Abstract
Bone grafting remains an essential component of reconstructive procedures in oral and maxillofacial surgery, orthopedics, and periodontics. The ongoing development and refinement of various bone graft materials have expanded treatment options while raising questions about their comparative efficacy and appropriate applications. This article provides a comprehensive analysis of contemporary bone grafting materials, examining their biological properties, clinical applications, advantages, and limitations. The comparative analysis encompasses autogenous bone, allografts, xenografts, and alloplastic materials, with particular emphasis on osteoconductive, osteoinductive, and osteogenic properties. Modern trends in combination grafts and growth factor enhancement are explored, along with a detailed case study demonstrating clinical decision-making in complex bone reconstruction. By evaluating the current evidence on various bone grafting materials, this review aims to provide clinicians with a framework for material selection based on specific clinical scenarios and desired outcomes.
, alloplast, osteoinduction, osteoconduction, biomaterials, bone regeneration
Introduction
Bone is the second most transplanted tissue worldwide, with only blood being transplanted more frequently (Finkemeier, 2002). Bone grafting procedures have become increasingly common across multiple disciplines, including dentistry, oral and maxillofacial surgery, orthopedics, neurosurgery, and plastic surgery. The fundamental biological concept underlying bone grafting involves providing a scaffold for new bone formation, stimulating bone-forming cells, and in some cases, directly supplying viable osteoblasts.
The ideal bone graft material would combine excellent biocompatibility, appropriate mechanical properties, predictable biological behavior, unlimited availability, and cost-effectiveness (Sheikh et al., 2015). While no single material currently satisfies all these criteria, the diversity of available options allows clinicians to select materials based on specific clinical requirements and constraints.
The historical “gold standard” of bone grafting materials has been autogenous bone, harvested from the patient’s own body. However, concerns regarding donor site morbidity, limited availability, and unpredictable resorption patterns have driven the development of alternative materials (Dimitriou et al., 2011). These alternatives include allografts (derived from human donors), xenografts (derived from other species), and alloplastic materials (synthetic substitutes).
Each category of bone grafting material exhibits unique biological, mechanical, and handling properties, with distinct advantages and limitations. Understanding these characteristics is crucial for making informed clinical decisions that optimize patient outcomes. This review aims to provide a comprehensive analysis of contemporary bone grafting materials, exploring their biological mechanisms, clinical applications, and relative efficacy based on current scientific evidence.
Biological Principles of Bone Regeneration
Before comparing specific graft materials, it is essential to understand the fundamental biological mechanisms through which bone grafts function:
Osteogenesis refers to the direct formation of new bone by living osteoblasts present within the graft material. Only autogenous bone grafts contain viable cells capable of this process, making them uniquely osteogenic (Albrektsson & Johansson, 2001).
Osteoinduction
Osteoinduction involves the stimulation of mesenchymal stem cells from the surrounding tissues to differentiate into osteoblasts, thereby initiating new bone formation. This process is mediated by growth factors, primarily bone morphogenetic proteins (BMPs), contained within the bone matrix (Urist, 1965). Autografts and some processed allografts possess osteoinductive properties.
Osteoconduction
Osteoconduction describes the process by which a graft material serves as a scaffold or framework for the ingrowth of capillaries, perivascular tissues, and osteoprogenitor cells from the recipient bed. Most bone graft materials, including autografts, allografts, xenografts, and alloplastic materials, provide some degree of osteoconduction (Bauer & Muschler, 2000).
Osseointegration
Osseointegration refers to the direct structural and functional connection between living bone and the surface of a load-bearing implant or graft material. This concept is particularly relevant when bone grafting is performed in conjunction with dental implant placement or orthopedic hardware (Brånemark et al., 1977).
Comparative Analysis of Bone Grafting Materials
Autogenous Bone Grafts
Autogenous bone, harvested from the patient’s own body, remains the benchmark against which other grafting materials are compared.
Biological Properties
Autografts are uniquely trimodal, combining osteogenic, osteoinductive, and osteoconductive properties (Khan et al., 2005). They contain viable osteoblasts and osteoprogenitor cells, growth factors embedded in the bone matrix, and a natural architecture that facilitates revascularization and cell migration.
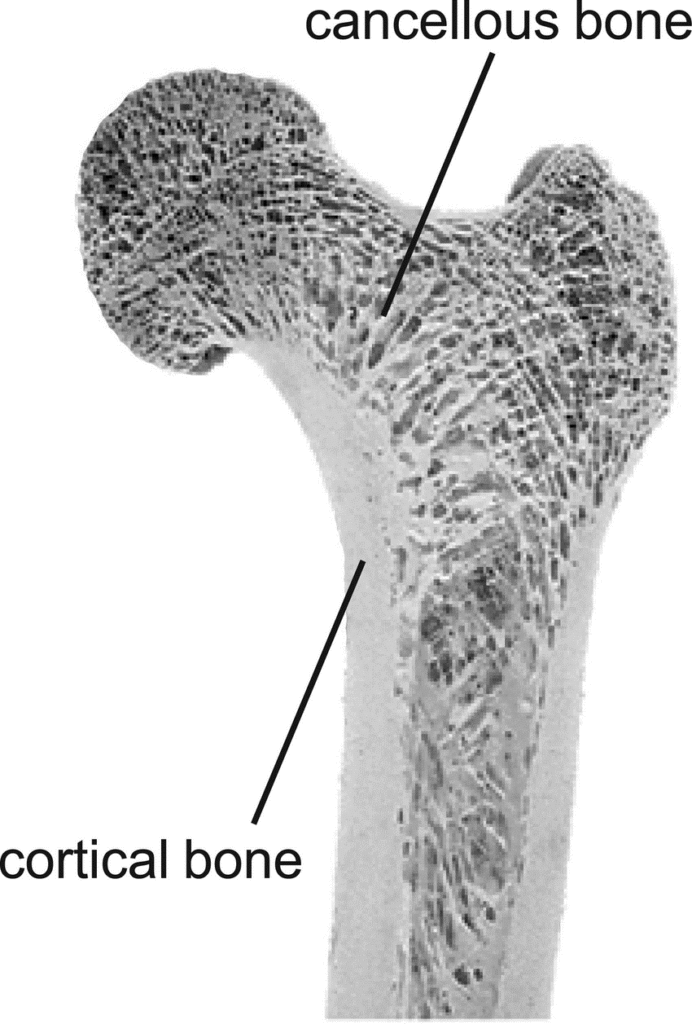
- Cancellous bone: Rich in cellular elements and highly vascular, cancellous bone grafts undergo rapid revascularization and incorporation but provide limited structural support. Common donor sites include the iliac crest, greater trochanter, proximal tibia, and in oral surgery, the mandibular ramus or symphysis (Misch, 1997).
- Cortical bone: Provides excellent structural integrity but revascularizes more slowly than cancellous bone. Cortical grafts are typically harvested from the mandibular ramus, tibial shaft, or fibula (Marx & Morales, 1988).
- Cortico-cancellous blocks: Combine the structural advantages of cortical bone with the biological benefits of cancellous bone. The anterior or posterior iliac crest is a common donor site for substantial cortico-cancellous grafts (Nkenke et al., 2004).
- Particulate bone: Can be harvested using bone scrapers, rongeurs, or bone mills. Particulate grafts are easily contoured to defect morphology but lack structural integrity (Urban et al., 2017).
Clinical Applications
Autografts are particularly valuable in:
- Reconstruction of large defects requiring structural support
- Sites with compromised vascularity
- Situations requiring enhanced osteogenic potential
- Two-stage implant placement in sites with severe atrophy
- Correction of congenital or post-traumatic deformities
Advantages
- Complete histocompatibility with no risk of disease transmission
- Presence of viable osteoprogenitor cells (osteogenic potential)
- Natural architecture optimized for bone regeneration
- Contains growth factors that promote bone formation
- Excellent incorporation and remodeling capabilities
Limitations
- Requires additional surgical site with associated morbidity
- Limited availability, particularly for large defects
- Unpredictable resorption rates, especially with cancellous grafts
- Increased operative time and patient discomfort
- Potential for donor site complications (pain, infection, fracture)
Allogeneic Bone Grafts (Allografts)
Allografts are derived from human donors, typically cadavers, and undergo extensive processing to reduce immunogenicity and eliminate disease transmission risk.
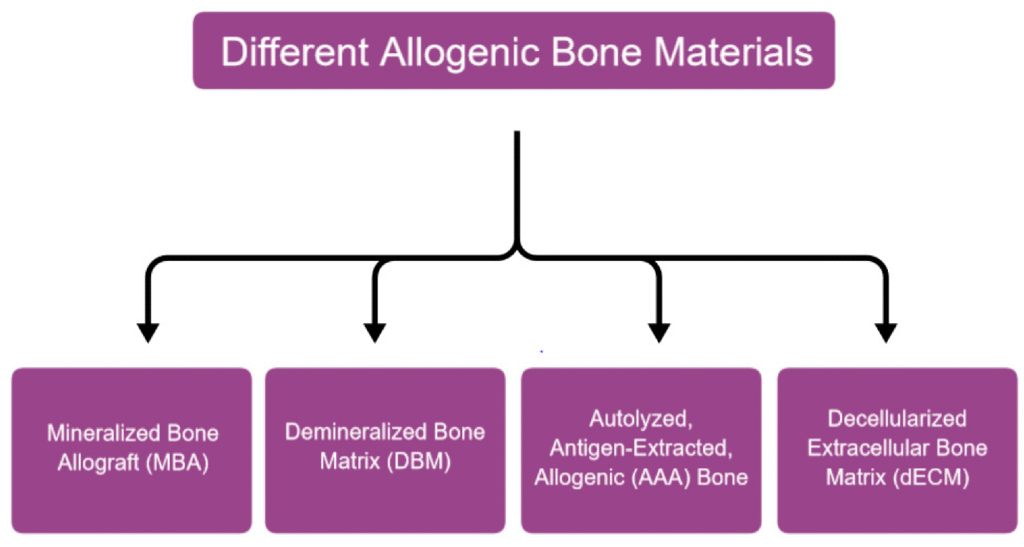
Types and Processing Methods
- Fresh-frozen allograft: Minimally processed and preserved by deep-freezing, retaining some growth factors but carrying higher immunogenic potential and disease transmission risk. Rarely used in contemporary practice except in specialized orthopedic applications (Stevenson, 1999).
- Freeze-dried bone allograft (FDBA): Processed by removing water through sublimation, reducing immunogenicity while preserving mechanical properties and some growth factors. Available in cortical, cancellous, or cortico-cancellous forms (Mellonig et al., 1981).
Biological Properties
Allografts are primarily osteoconductive, serving as scaffolds for new bone formation. DFDBA additionally exhibits osteoinductive properties, though to a lesser extent than autografts. Allografts lack viable cells and therefore have no osteogenic potential (Bauer & Muschler, 2000).
Clinical Applications
Allografts are commonly used in:
- Periodontal regeneration procedures
- Socket preservation following tooth extraction
- Ridge augmentation for implant site development
- Sinus floor elevation procedures
- Reconstruction of skeletal defects in orthopedic surgery
Advantages
- No donor site morbidity
- Unlimited availability in various forms and sizes
- Reduced surgical time compared to autograft harvesting
- Potential osteoinductive properties (particularly with DFDBA)
- Availability in structural forms (e.g., cortical struts, osseous discs)
Limitations
- Potential for immune response (though minimal with proper processing)
- Theoretical risk of disease transmission (extremely low with current screening)
- Variable osteoinductive potential between donors and processing techniques
- Slower incorporation than autografts
- Higher cost compared to some synthetic alternatives
Xenogeneic Bone Grafts (Xenografts)
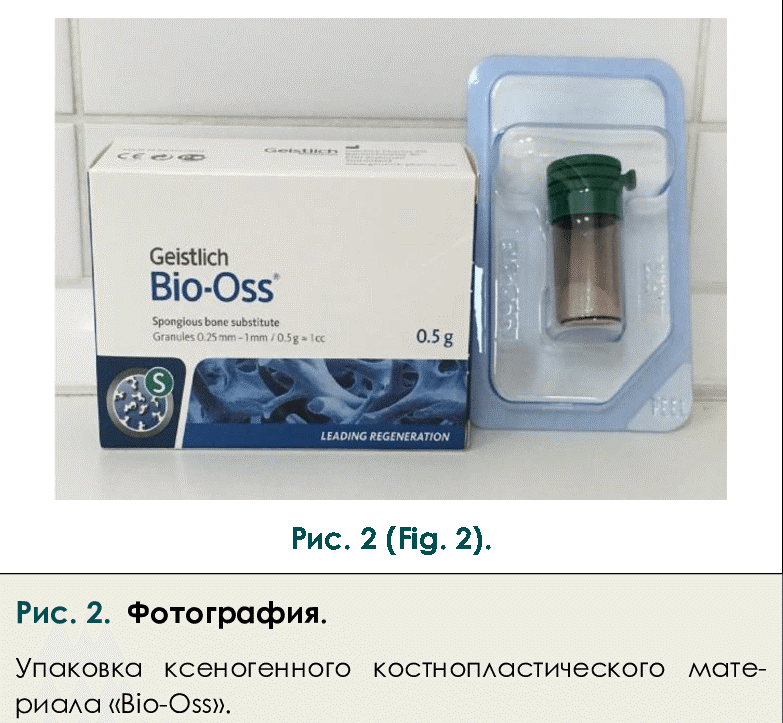
Xenografts are derived from non-human species, with bovine and porcine sources being the most common in clinical practice.
Types and Sources
- Bovine-derived xenografts: The most extensively studied and widely used, typically derived from bovine cancellous bone. The organic components are removed through various processing methods, leaving a natural calcium phosphate scaffold (Scarano et al., 2006).
- Porcine-derived xenografts: Similar to bovine materials but with potentially different architectural characteristics. Some evidence suggests physicochemical properties closer to human bone (Nannmark & Sennerby, 2008).
- Equine-derived xenografts: Processed through enzymatic deantigenation or high-temperature treatment to remove organic components while preserving the natural structure (Di Stefano et al., 2009).
- Coral-derived materials: Natural coral exoskeletons composed of calcium carbonate may be used either in their native form or hydrothermally converted to hydroxyapatite (Guillemin et al., 1987).
Biological Properties
Xenografts are exclusively osteoconductive, serving as scaffolds for new bone formation. They lack osteogenic cells and contain no viable osteoinductive factors after processing. The slow resorption rate of most xenografts provides long-term space maintenance, which can be advantageous in certain clinical scenarios (Jensen et al., 2006).
Clinical Applications
Xenografts are particularly valuable in:
- Sinus floor elevation procedures
- Ridge preservation following extraction
- Horizontal ridge augmentation in combination with barrier membranes
- Periodontal defect regeneration
- Filling of cystic cavities
Advantages
- No donor site morbidity
- Unlimited availability
- Natural architecture similar to human bone
- Excellent volume maintenance due to slow resorption
- Extensive documentation in the scientific literature
Limitations
- Lack of osteogenic and osteoinductive properties
- Potential ethical or religious concerns for some patients
- Prolonged presence in the grafted site (may be advantage or disadvantage)
- Variable physical properties depending on source and processing
- Complete resorption may never occur with some products
Alloplastic Bone Graft Substitutes
Alloplastic materials are synthetic bone substitutes manufactured from various combinations of calcium phosphates, bioactive glass, calcium sulfate, or polymers.
Types and Compositions
- Calcium phosphate ceramics: Include hydroxyapatite (HA), beta-tricalcium phosphate (β-TCP), and biphasic calcium phosphate (BCP). These materials mimic the mineral phase of natural bone and vary in their resorption rates and mechanical properties (LeGeros, 2008).
- Bioactive glass: Composed of silicon dioxide, sodium oxide, calcium oxide, and phosphorus pentoxide in specific proportions. Forms a hydroxycarbonate apatite layer when exposed to biological fluids, promoting strong bonding with bone and soft tissues (Hench, 2006).
- Calcium sulfate: One of the oldest synthetic bone substitutes, provides minimal structural support but resorbs completely within 4-12 weeks, being replaced by new bone (Thomas & Puleo, 2009).
- Polymer-based materials: Include polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers. Offer controllable degradation rates and the potential for drug delivery applications (Nair & Laurencin, 2007).
- Composite materials: Combine multiple components to optimize biological and mechanical properties, such as HA/TCP with collagen or bioactive glass with polymeric matrices (Rezwan et al., 2006).
Biological Properties
Alloplastic materials are primarily osteoconductive, providing a scaffold for bone cell attachment and growth. They lack intrinsic osteoinductive or osteogenic properties but can be modified to incorporate growth factors or bioactive molecules to enhance their biological performance (Habibovic & de Groot, 2007).
Clinical Applications
Alloplastic materials are commonly used in:
- Filling of periodontal defects
- Ridge preservation following tooth extraction
- Sinus floor elevation procedures
- Non-load-bearing craniofacial defects
- Orthopedic applications as bone void fillers
Advantages
- No risk of disease transmission
- Unlimited availability
- Controllable physical and chemical properties
- Variety of forms (blocks, granules, putties, injectable)
- Potential for tailored resorption rates
Limitations
- Lack of osteogenic and osteoinductive properties
- Variable integration with host bone
- Potential for particle migration or dislocation
- Limited application in load-bearing situations
Comparative Efficacy: Evidence from Clinical Studies
Ridge Preservation and Socket Grafting
Multiple systematic reviews have compared various graft materials for alveolar ridge preservation following tooth extraction. Vignoletti et al. (2012) concluded that while all materials reduced dimensional changes compared to ungrafted sockets, xenografts demonstrated the most favorable outcomes for horizontal ridge preservation. Iocca et al. (2017) similarly found that xenografts and alloplasts outperformed allografts in maintaining ridge dimensions, though all materials showed clinical efficacy.
Sinus Floor Elevation
In a systematic review comparing different graft materials for maxillary sinus augmentation, Danesh-Sani et al. (2016) reported comparable implant survival rates across autografts, allografts, xenografts, and alloplastic materials. However, autografts demonstrated the highest rate of new bone formation (47%), followed by xenografts (30%), allografts (29.5%), and alloplastic materials (24%). Starch-Jensen et al. (2018) similarly concluded that all materials yielded satisfactory long-term outcomes, though autografts remained slightly superior in terms of bone quality and quantity.
Horizontal Ridge Augmentation
For horizontal ridge augmentation, autogenous block grafts have historically shown superior results. Urban et al. (2017) demonstrated successful outcomes using a combination of particulated autograft and xenograft protected by titanium-reinforced membranes. Troeltzsch et al. (2016), in a systematic review, concluded that autogenous block grafts yielded the greatest horizontal gain (4.25mm), followed by guided bone regeneration using particulate grafts (3.61mm).
Vertical Ridge Augmentation
Vertical augmentation represents one of the most challenging scenarios in bone regeneration. Rocchietta et al. (2008) reviewed the literature and found that autogenous blocks, distraction osteogenesis, and guided bone regeneration with particulate grafts could all achieve vertical gains, though autografts demonstrated the most predictable outcomes. Urban et al. (2019) reported successful vertical augmentation using a composite graft of particulated autogenous bone and anorganic bovine bone mineral.
Modern Trends and Advancements
Combination Grafts
Recognizing the limitations of individual materials, many clinicians now employ combination grafts to harness complementary properties:
- Autograft + xenograft: Combines the osteogenic potential of autogenous bone with the volume stability of xenografts. Urban et al. (2017) reported successful outcomes using a 1:1 ratio for large augmentations.
- Allograft + xenograft: Offers potentially enhanced osteoinductivity with prolonged space maintenance. Avila-Ortiz et al. (2016) demonstrated favorable results using this combination for socket preservation.
- Layered approaches: Utilizing different materials in specific locations within the defect. For example, placing autografts in direct contact with the recipient bed to enhance vascularization, with slower-resorbing materials in the peripheral aspects (Wang & Boyapati, 2006).
Growth Factor Enhancement
Various bioactive molecules have been employed to enhance the regenerative potential of bone grafting materials:
- Platelet concentrates: Both platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) have been used to deliver autologous growth factors to grafted sites. While results are variable, meta-analyses suggest modest benefits in early healing phases (Del Fabbro et al., 2011).
- Bone morphogenetic proteins (BMPs): Particularly rhBMP-2 and rhBMP-7, have demonstrated significant osteoinductive potential. FDA-approved applications include spinal fusion, tibial non-unions, and maxillary sinus augmentation (McKay et al., 2007).
- Peptide modifications: Short peptide sequences that mimic extracellular matrix proteins, such as RGD (arginine-glycine-aspartic acid), have been incorporated into graft materials to enhance cell adhesion and differentiation (Kim et al., 2016).
3D-Printed Custom Scaffolds
Advances in additive manufacturing have enabled the production of patient-specific bone graft substitutes:
- Controlled internal architecture: Facilitating optimal vascularization and cellular infiltration through precisely designed pore size, orientation, and interconnectivity (Karageorgiou & Kaplan, 2005).
- Multi-material printing: Allowing for gradient structures that mimic the cortico-cancellous architecture of natural bone (Di Bella et al., 2018).

Clinical Decision-Making: Material Selection Framework
The selection of appropriate bone grafting materials should be guided by a systematic evaluation of multiple factors:
- Defect characteristics: Size, morphology, number of walls, load-bearing requirements
- Host factors: Age, systemic health, smoking status, medication use
- Surgical goals: Immediate vs. delayed implant placement, expected timeframe for healing
- Functional demands: Aesthetic vs. posterior region, occlusal forces
- Patient preferences: Acceptance of additional surgical sites, religious or ethical concerns
- Clinical setting: Available facilities, surgical expertise, cost considerations
Case Study: Complex Anterior Maxillary Reconstruction
Patient Information
A 45-year-old female patient presented with a missing right central incisor (#8) following traumatic avulsion 12 months prior. The patient had no significant medical history and was a non-smoker.
Clinical and Radiographic Assessment
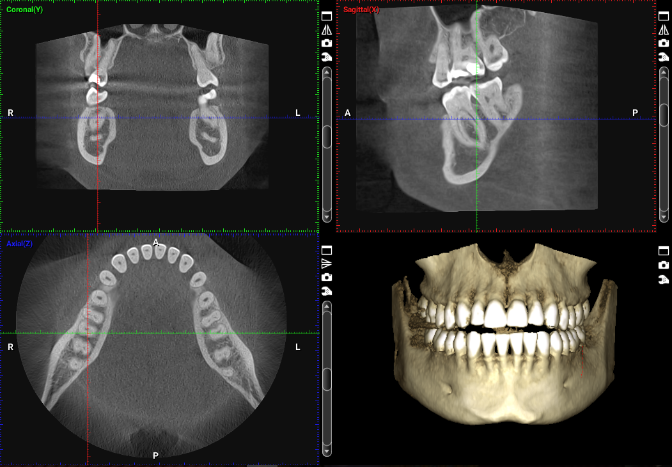
Clinical examination revealed severe horizontal and vertical deficiency of the alveolar ridge in the #8 region, with measurements of 3.5mm in width and 7mm vertical deficiency compared to adjacent teeth. CBCT analysis confirmed these findings and demonstrated the three-dimensional nature of the defect, with significant labial concavity and compromised ridge height.
Treatment Planning Considerations
The anterior location demanded exceptional aesthetic outcomes, requiring comprehensive reconstruction of both hard and soft tissues. The complex three-dimensional defect necessitated a grafting approach that would provide both structural support and reliable volume stability. The patient declined autogenous bone harvesting from extraoral sites but was amenable to intraoral donor sites.
Material Selection Rationale
A combination grafting approach was selected based on the following considerations:
- Autogenous bone block from the mandibular symphysis would provide:
- Structural support for the vertical component
- Osteogenic cells to accelerate healing
- Superior integration at the recipient site
- Particulate xenograft (Bio-Oss, Geistlich Pharma) would offer:
- Long-term volume stability
- Prevention of resorption of the autogenous block
- Favorable soft tissue response
- A titanium-reinforced non-resorbable membrane (Cytoplast Ti-250, Osteogenics Biomedical) was selected to:
- Ensure space maintenance
- Prevent soft tissue ingrowth
- Allow extended healing time
Surgical Procedure
The remaining spaces around the block were filled with a mixture of autogenous bone chips collected during site preparation and particulate xenograft in a 1:1 ratio. The entire grafted area was covered with the titanium-reinforced membrane, which was secured with tacks.
Periosteal releasing incisions allowed tension-free primary closure with 5-0 non-resorbable sutures.
Healing Period and Second-Stage Surgery
A six-month healing period was observed, during which the patient wore a provisional removable partial denture modified to avoid pressure on the surgical site.
At six months, CBCT evaluation demonstrated successful integration of the graft, with adequate bone volume for implant placement. Second-stage surgery involved membrane removal, implant placement (4.3 × 12mm, Straumann Bone Level), and simultaneous connective tissue grafting to enhance soft tissue volume.
Following a three-month osseointegration period, the implant was restored with a screw-retained all-ceramic crown.
Outcome and Follow-up
At the one-year follow-up appointment, clinical and radiographic examination revealed stable peri-implant tissues, appropriate bone levels, and an aesthetically pleasing result. The combination grafting approach successfully reconstructed the complex defect, providing both the structural framework and long-term volume stability required for an aesthetic outcome.
Case Discussion
This case illustrates several important principles in bone graft material selection:
- Complementary properties: The combination of autogenous bone (providing osteogenic cells and structural support) with xenograft (offering volume stability) addressed the multifaceted requirements of the defect.
- Site-specific considerations: The aesthetic zone demanded both functional and aesthetic excellence, influencing the selection of materials known for predictable outcomes and favorable tissue response.
- Patient factors: Willingness to undergo intraoral but not extraoral harvesting guided the selection of the donor site.
- Long-term stability: The incorporation of slowly resorbing xenograft particles around the autogenous block aimed to prevent the well-documented resorption of autografts over time.
This case demonstrates that thoughtful material selection based on scientific principles and case-specific requirements can yield optimal outcomes in challenging clinical scenarios.
Conclusion
The comparative analysis presented in this review demonstrates that no single material universally outperforms all others across all clinical applications. Rather, each category exhibits unique strengths and limitations that may be advantageous in specific contexts. Consequently, material selection should be guided by a systematic evaluation of defect characteristics, host factors, surgical goals, and practical considerations.
Contemporary approaches increasingly utilize combination grafts to harness complementary properties and overcome the limitations of individual materials. Additionally, ongoing research in growth factor enhancement and custom scaffold fabrication continues to expand the frontiers of bone regeneration.
As our understanding of bone biology advances and biomaterial science evolves, the ideal bone graft substitute—combining excellent biocompatibility, appropriate mechanical properties, predictable biological behavior, unlimited availability, and cost-effectiveness—may eventually become a reality. Until then, clinicians must remain informed about the comparative properties of available materials to make evidence-based decisions that optimize patient outcomes.
References
- Albrektsson, T., & Johansson, C. (2001). Osteoinduction, osteoconduction and osseointegration. European Spine Journal, 10(Suppl 2), S96-S101.
- Avila-Ortiz, G., Elangovan, S., Kramer, K. W., Blanchette, D., & Dawson, D. V. (2016). Effect of alveolar ridge preservation after tooth extraction: a systematic review and meta-analysis. Journal of Dental Research, 93(10), 950-958.
- Bauer, T. W., & Muschler, G. F. (2000). Bone graft materials: an overview of the basic science. Clinical Orthopaedics and Related Research, 371, 10-27.
- Brånemark, P. I., Hansson, B. O., Adell, R., Breine, U., Lindström, J., Hallén, O., & Ohman, A. (1977). Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scandinavian Journal of Plastic and Reconstructive Surgery. Supplementum, 16, 1-132.
- Danesh-Sani, S. A., Engebretson, S. P., & Janal, M. N. (2016). Histomorphometric results of different grafting materials and effect of healing time on bone maturation after sinus floor augmentation: a systematic review and meta-analysis. Journal of Periodontal Research, 52(3), 301-312.
- Del Fabbro, M., Bortolin, M., Taschieri, S., & Weinstein, R. (2011). Is platelet concentrate advantageous for the surgical treatment of periodontal diseases? A systematic review and meta-analysis. Journal of Periodontology, 82(8), 1100-1111.
- Di Bella, C., Duchi, S., O’Connell, C. D., Blanchard, R., Augustine, C., Yue, Z., Thompson, F., Richards, C., Beirne, S., Onofrillo, C., Bauquier, S. H., Ryan, S. D., Pivonka, P., Wallace, G. G., & Choong, P. F. (2018). In situ handheld three-dimensional bioprinting for cartilage regeneration. Journal of Tissue Engineering and Regenerative Medicine, 12(3), 611-621.
- Di Stefano, D. A., Artese, L., Iezzi, G., Piattelli, A., Pagnutti, S., Piccirilli, M., & Perrotti, V. (2009). Alveolar ridge regeneration with equine spongy bone: a clinical, histological, and immunohistochemical case series. Clinical Implant Dentistry and Related Research, 11(2), 90-100.
- Dimitriou, R., Mataliotakis, G. I., Angoules, A. G., Kanakaris, N. K., & Giannoudis, P. V. (2011). Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury, 42(Suppl 2), S3-S15.
- Finkemeier, C. G. (2002). Bone-grafting and bone-graft substitutes. The Journal of Bone and Joint Surgery. American Volume, 84(3), 454-464.
- Guillemin, G., Patat, J. L., Fournie, J., & Chetail, M. (1987). The use of coral as a bone graft substitute. Journal of Biomedical Materials Research, 21(5), 557-567.
- Habibovic, P., & de Groot, K. (2007). Osteoinductive biomaterials—properties and relevance in bone repair. Journal of Tissue Engineering and Regenerative Medicine, 1(1), 25-32.
- Hench, L. L. (2006). The story of Bioglass®. Journal of Materials Science: Materials in Medicine, 17(11), 967-978.
- Iocca, O., Farronato, D., Iezzi, G., & Piattelli, A. (2017). Alveolar ridge preservation after tooth extraction: a Bayesian Network meta-analysis of grafting materials efficacy on prevention of bone height and width reduction. Journal of Clinical Periodontology, 44(1), 104-114.
- Jensen, S. S., Broggini, N., Hjørting‐Hansen, E., Schenk, R., & Buser, D. (2006). Bone healing and graft resorption of autograft, anorganic bovine bone and β‐tricalcium phosphate. A histologic and histomorphometric study in the mandibles of minipigs. Clinical Oral Implants Research, 17(3), 237-243.
- Karageorgiou, V., & Kaplan, D. (2005). Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials, 26(27), 5474-5491.
- Khan, S. N., Cammisa Jr, F. P., Sandhu, H. S., Diwan, A. D., Girardi, F. P., & Lane, J. M. (2005). The biology of bone grafting. Journal of the American Academy of Orthopaedic Surgeons, 13(1), 77-86.
- Kim, B. S., Yang, S. S., & Lee, J. (2016). Precoating of biphasic calcium phosphate bone substitute with atelocollagen enhances bone regeneration through stimulation of osteoclast activation and angiogenesis. Journal of Biomedical Materials Research Part A, 104(4), 788-796.
- LeGeros, R. Z. (2008). Calcium phosphate-based osteoinductive materials. Chemical Reviews, 108(11), 4742-4753.
- Marx, R. E., & Morales, M. J. (1988). Morbidity from bone harvest in major jaw reconstruction: a randomized trial comparing the lateral anterior and posterior approaches to the ilium. Journal of Oral and Maxillofacial Surgery, 46(3), 196-203.
- McKay, W. F., Peckham, S. M., & Badura, J. M. (2007). A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE® Bone Graft). International Orthopaedics, 31(6), 729-734.
- Mellonig, J. T., Bowers, G. M., & Bailey, R. C. (1981). Comparison of bone graft materials: Part I. New bone formation with autografts and allografts determined by Strontium-85. Journal of Periodontology, 52(6), 291-296.
- Misch, C. E. (1997). Comparison of intraoral donor sites for onlay grafting prior to implant placement. International Journal of Oral & Maxillofacial Implants, 12(6), 767-776.
- Nair, L. S., & Laurencin, C. T. (2007). Biodegradable polymers as biomaterials. Progress in Polymer Science, 32(8-9), 762-798.
- Nannmark, U., & Sennerby, L. (2008). The bone tissue response to prehydrated and collagenated cortico-cancellous porcine bone grafts: a study in rabbit maxillary defects. Clinical Implant Dentistry and Related Research, 10(4), 264-270.
- Nkenke, E., Weisbach, V., Winckler, E., Kessler, P., Schultze-Mosgau, S., Wiltfang, J., & Neukam, F. W. (2004). Morbidity of harvesting of bone grafts from the iliac crest for preprosthetic augmentation procedures: a prospective study. International Journal of Oral & Maxillofacial Surgery, 33(2), 157-163.
- Rezwan, K., Chen, Q. Z., Blaker, J. J., & Boccaccini, A. R. (2006). Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials, 27(18), 3413-3431.
- Rocchietta, I., Fontana, F., & Simion, M. (2008). Clinical outcomes of vertical bone augmentation to enable dental implant placement: a systematic review. Journal of Clinical Periodontology, 35(8 Suppl), 203-215.
- Scarano, A., Degidi, M., Iezzi, G., Pecora, G., Piattelli, M., Orsini, G., Caputi, S., Perrotti, V., Mangano, C., & Piattelli, A. (2006). Maxillary sinus augmentation with different biomaterials: a comparative histologic and histomorphometric study in man
